Connect2HealthFCC - Wireless Health and Medical Devices Background
The FCC's Office of Engineering and Technology (OET) conducts many activities relating to wireless medical devices, including equipment authorization (for products such as smartphones, car door remote controls, Wi-Fi devices, baby monitors, and personal computers); testing for radio frequency safety; and regulation of radio spectrum.
OET has a long history of working to enable health and medical devices. Some of its more recent accomplishments include:
MedRadio (Medical Device Radiocommunications Service): In the course of two rulemaking proceedings, the Commission allocated spectrum and adopted technical rules for innovative new body-worn and implanted medical radio devices that can provide a variety of diagnostic and therapeutic functions from diabetes and heart monitors to pacemakers and cardiofribrilators.
Medical Body Area Networks (MBANs): In 2012, the FCC released an Order to allocate spectrum for Medical Body Area Networks (MBANs), making the U.S. the first country in the world to make spectrum available for this specific usage. MBANs are networks of wireless sensors, often no bigger than a Band-Aid, which can transmit data on a patient's vital health indicators to their doctor or hospital. For more information, see the MBANs Fact Sheet and a listing of Frequency Bands for Medical Devices.
Medical Micropower Networks (MMNs): In 2011, the FCC adopted rules to enable a new generation of wireless medical devices that can be used to restore functions to paralyzed limbs. MMNs are ultra-low power wideband networks consisting of transmitters implanted in the body that take the place of damaged nerves, restoring sensation and mobility.
Retinal Implants: In November 2011, OET granted a waiver of the Commission's rules to Second Sight Medical Products, Inc. to allow it to obtain FCC certification for and market its Argus II Retinal Prosthesis System which is a medical implant system designed to treat profoundly blind people suffering from advanced retinal degenerative diseases. For more information, see the waiver (https://docs.fcc.gov/public/attachments/DA-11-1951A1.pdf)
Experimental Licensing Program: In May 2012, the FCC announced a plan to cut red tape and increase spectrum flexibility for testing new wireless health innovations, to speed new wireless health technologies to market. The new experimental licensing regime will create more flexibility and streamlined processes for testing new wireless medical devices.
FCC-FDA Memorandum of Understanding: In 2010, the FCC entered into an unprecedented partnership with the Food and Drug Administration, working together to ensure that communications-related medical innovations can swiftly and safely be brought to market. In June 2012, the FCC issued a letter in response to an inquiry from Reps. Walden, Bilbray, Blackburn, Burgess, Gingrey, and Pitts regarding wireless medical devices and the FCC's partnership with FDA, providing a comprehensive overview of activities that the two agencies have undertaken since enacting the MOU in 2010.
Frequency Bands for Medical Devices
Medical Radio Communications Service (MedRadio) Authorized under Part 95, subpart I
401-406 MHz Frequency Band – General Usage: medical devices for transmitting data containing operational, diagnostic and therapeutic information associated with a medical implant device or medical body worn devices
Frequency Bands for Specific Applications:
Medical Micropower Networks (MMNs): wireless medical devices that can be used to restore functions to paralyzed limbs
- 413-419 MHz
- 426-432 MHz
- 438-444 MHz
- 451-457 MHz
2360-2400 MHz Medical Body Area Networks (MBANs): networks of body-worn wireless sensors that transmit patient data to a health care provider
Please refer to the rules to determine specific technical and operational rules for each MedRadio frequency band. Wireless Medical Telemetry Service (WMTS): a short distance data communication service for transmitting patient medical information to a central monitoring location in a medical facility
Authorized under Part 95, subpart H
Frequency Bands:
- 608-614 MHz
- 1395-1400 MHz
- 1427-1429.5 MHz(location specific)
- 1429-1431.5 MHz (location specific)
Please refer to the rules to determine specific technical and operational rules for each MedRadio frequency band. Medical devices may also operate under the rules for unlicensed devices under Part 15 in any frequency band available under that Part.
IMPLANTS


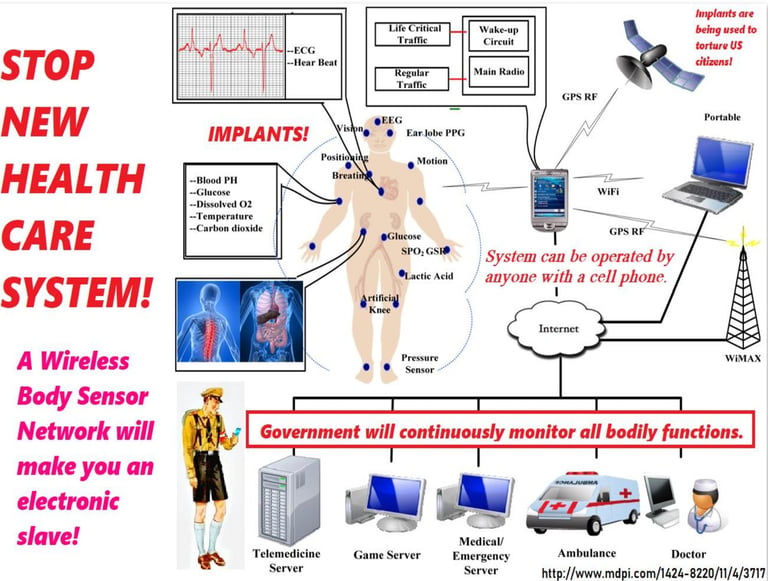


Nano-Implants for Wireless Brain Interfacing
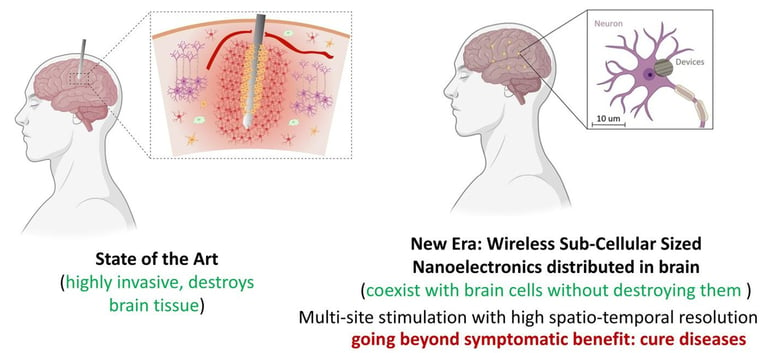
We are developing nano-devices using meta-materials that can non-invasively and remotely monitor and modulate our biological system. The requirements of the system are: 1> they should be as small as possible such that the volume displacement of tissue due to the placement of the device is minimal, 2> They should be untethered/wireless such that they can be remotely controlled. Such a device will sense the biological environment and send the information to a system outside the body in real time. The device will also have the capability to do internal analysis of the sensed data and depending on the analysis results, take further action such as electrical stimulation or drug delivery. The device will harvest energy from external applied fields for its functioning and also modulate the external fields for communicating sensed data.
The possibilities with such bioelectronic devices are endless, and we are exploring, among others, brain activity recording at a large scale with a precision of single neuron, activity recording in spinal cord and peripheral nervous system, monitoring tumor microenvironment, observing response to pathology development or external stimulus at a single cell level along with integrated functionalities such as stimulation and drug delivery.
https://www.media.mit.edu/projects/wireless-sensing/overview/
Osseosurface electronics
One emerging application of implantable wireless sensors is osseosurface electronics, an implementation in which wireless sensing devices are implanted and adhered directly to bone surfaces [168]. These battery-free devices are equipped with multimodal capabilities, including sensors for real-time bone strain measurement, millikelvin resolution thermography, and optical stimulation. They operate wirelessly through energy harvesting and data transmission, eliminating the need for internal batteries. The devices are permanently bonded to the bone using calcium phosphate ceramic particles, demonstrating stable integration and functionality in deep tissue environments. The system features a hybrid integration of a flexible substrate with miniaturized components for analog and digital functions, enabling conformal attachment to the bone while maintaining stability and minimizing strain on the target sensing region. The device utilizes magnetic resonant coupling (13.56 MHz) for wireless, battery-free power harvesting through thick tissues up to 11.5 cm. The system operates using NFC-compatible hardware, featuring a compact NFC system-on-chip (4 mm × 4 mm) that integrates a microcontroller and transponder for operational control (Fig. 7A). The analog front-ends (AFE) for strain and temperature sensing are optimized for low power consumption and high sensitivity.
https://link.springer.com/article/10.1007/s44258-024-00041-3#citeas
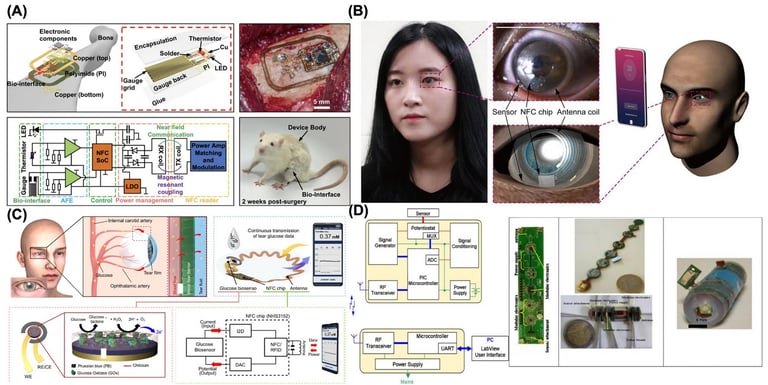
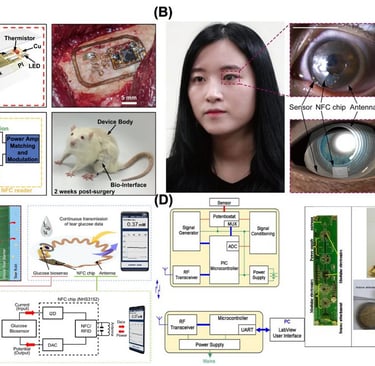


Technologies and applications in wireless biosensors for real-time health monitoring
Published: 25 November 2024
Xu, Z., Hao, Y., Luo, A. et al. Technologies and applications in wireless biosensors for real-time health monitoring. Med-X 2, 24 (2024). https://doi.org/10.1007/s44258-024-00041-3
Implantable Sensors:
Wireless implantable, ingestible, or digestible biosensors allow for rigorous biophysical and biochemical monitoring, enabling real-time closed-loop interventions through in vivo measurements and continuous physiological detection [166]. Several biomedical applications exist for implantable sensors, ranging from neurological to gastroenterological pathways [167].

Technical and Feasibility Analysis
Power Discrepancy: WBAN energy harvesting devices are designed to generate minute amounts of power, typically in the milliwatt range, which is just enough to power low-energy medical sensors or recharge small internal batteries. In contrast, a single modern Bitcoin mining machine (ASIC) consumes thousands of watts (kilowatts) of power, an amount equivalent to several households.
Energy Sources: The energy sources used for WBANs are typically body movements (kinetic) or body heat (thermal), which are low-density and highly variable.
Computational Intensity: Cryptocurrency mining, particularly for Proof-of-Work systems like Bitcoin, relies on immense computational effort and the associated massive energy consumption to solve complex mathematical problems. The limited energy from WBANs cannot power the specialized, high-performance hardware needed for this task.
Related Concepts:
While direct energy harvesting for standard crypto mining is not practical, some related concepts have been explored in research and art projects:
Proof-of-Activity Patents: A Microsoft patent application from 2020 described a theoretical system where human body activity (like brain waves or body heat emitted when performing a task such as viewing an advertisement) could be used as "proof-of-work" to verify data and earn cryptocurrency, rather than using traditional intensive computation. This system uses the data of activity as proof, not the energy itself to power the computational process.
Art Projects: An art project by the Dutch "Institute of Human Obsolescence" (IoHO) in 2018 used special suits to capture the body heat from 37 people to power a small computer that mined a negligible amount of cryptocurrency over several hours. This was primarily a symbolic project to highlight the link between human labor and data production.
In summary, the power available from WBANs is fundamentally insufficient for the energy demands of cryptocurrency mining.
Performance Evaluation of Magnetic Resonance Coupling Method for Intra-Body Network (IBNet)
[I do not have the full document on this one.] THEORETICAL RESEEARCH!
Spurred by the advances in ultra-low-power electronics and communications, emerging implantable medical devices have led to new insights into long-term and continuous healthcare monitoring. For effective management of such medical devices, the intrabody network (IBNet) is becoming increasingly important. However, traditional means of intra-body communication (e.g., galvanic, capacitive, RF) are often affected by significant path loss due to tissue absorption, shadowing effect, environmental variations, instability of transmission quality, grounding issues, antenna size, etc. In pursuit of more suitable technology for the IBNet, the magnetic resonance (MR) coupling arose a great interest in the community since the magnetic permeability of the biological tissue is similar to that of the air, absence of an external reference or ground, and near-field operation.
In this paper, we present magnetic resonance (MR) coupling as a promising method for the intra-body network (IBNet). With seven healthy human subjects, we systematically compared MR coupling to traditional intra-body communication methods (galvanic, capacitive, and RF). The study revealed that MR coupling could effectively send or receive signals (power/data) in biological tissue, with a maximum path loss (PL) less than 33 dB (i.e., at 13.56 MHz). Such path loss was lower than galvanic, capacitive, or RF couplings for the same distance. The angular orientation between the transmitter and the receiver coils showed minor variation in the path loss (0.19 ≤ ∆PL ≤ 0.62 dB) but observed some dependency on the distance (0.05 dB/cm). Different postures during the MR coupling essentially did not affect performance (∆PL ≤ 0.21 dB). The multi-nodal transmission between a single transmitter and multiple receivers showed that the signal could be simultaneously delivered, demonstrating a potential for communication, sensing, and powering wearable and implantable devices. Overall, the MR coupling conserves energy for long-term implants by enabling low-power communication at lower path loss in the human body. https://www.embs.org/featured-articles/performance-evaluation-of-magnetic-resonance-coupling-method-for-intra-body-network-ibnet/
Magnetic Resonance Coupling for Intra Body Network (1BNet)
Mining your body's energy for Crypto Currency? [THEORETICAL]

Join us in advocating against harmful technology misuse.
contact@targetedhumans.org
© 2025. All rights reserved.
Targeted Humans Inc.